A Hole in the Ozone Layer
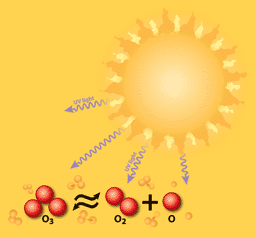
Balancing Act: O3 ↔ O2 + O
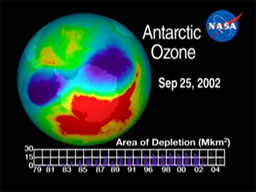
About 20 years ago, scientists realized that the ozone layer over Antarctica grew thinner during the winter and spring. This thinning became known as the ozone hole. It resulted from chemicals called chlorofluorocarbons (CFCs) used in aerosol spray cans and refrigerators.
In the stratosphere, oxygen atoms are constantly changing from ozone (three atoms) to oxygen gas (two atoms) and atomic oxygen (one atom) and back. By the 1970s, enough CFCs had reached the stratosphere to disrupt the ozone/oxygen balance. The ozone hole appeared over Antarctica, because cold air creates clouds of ice crystals that accelerate ozone loss.
The production of CFCs was phased out around the world starting in 1995. The ozone hole has stopped growing, but it’s not expected to close completely for another 40 or 50 years at best. Until then, more UV light will reach all parts of Earth, and people will have to be more careful about protecting themselves from sunlight.
Poor Plankton
Plankton (tiny plants and animals in the ocean) grow poorly or die when exposed to excessive UV radiation. So who cares if plankton can’t grow? Plankton sustain the oceans’ food chains, which culminate in whales and tuna. So if you care about whales and tuna, you should care about plankton too.
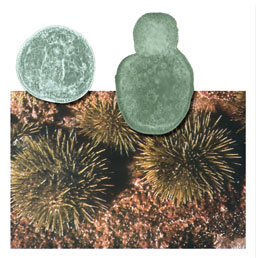
- Against the background of a mature sea urchin (Strongylocentrotus droebachiensis) are two sea urchin embryos. One is normal, and one was deformed by over-exposure to UV radiation. Can you tell which is which?
Answer: the embryo on the right received too much UV light. - Photos © Nikki Adams